How Do Countries Create Nuclear Weapons?
What is nuclear power and how can the same process that generates nuclear energy create a nuclear bomb?
Understanding where nuclear materials come from and how they are enriched can help clarify the difficulties in preventing countries from turning raw materials into powerful weapons.
Where does nuclear material come from?
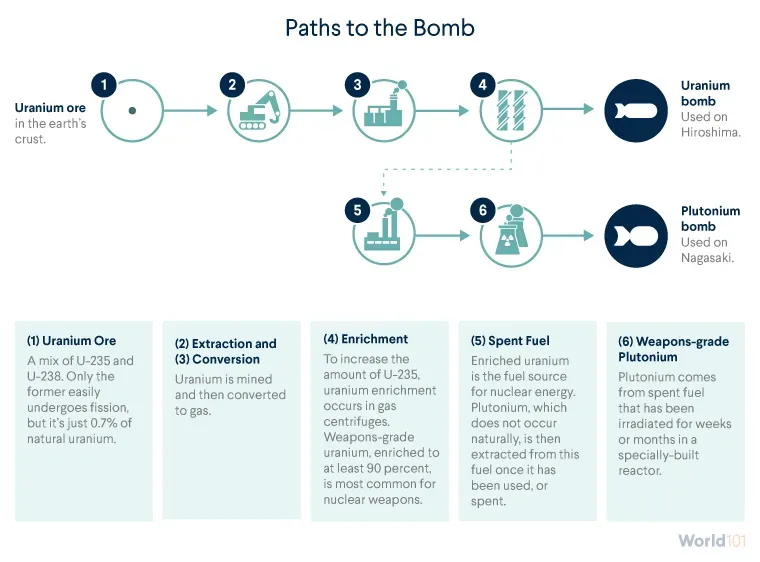
It all starts in the earth’s crust, home to uranium ore. Uranium is found all over the world, though mostly in trace quantities. Five countries—Australia, Canada, Kazakhstan, Namibia, and Russia—possess two-thirds of the world’s known supply.
Uranium is mined from the earth and then converted to gas so it can be enriched for nuclear purposes—either peaceful or destructive.
What is nuclear fission?
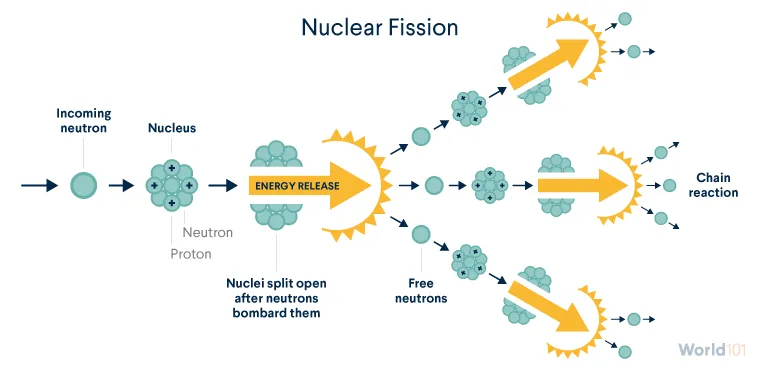
Fission is one of the scientific processes that helps create nuclear energy.
Nuclear fission occurs when neutrons bombard atomic nuclei and split them, releasing massive amounts of energy. Fission occurs easily in only a few isotopes, or forms of elements, typically uranium and plutonium. (Unlike uranium, plutonium isn’t found in nature—more on this issue later.)
In nature, uranium is a mix of mostly two isotopes: uranium-235 (U-235) and uranium-238 (U-238). U-235 is especially important because it easily undergoes fission (unlike U-238) and is extremely rare, accounting for less than 1 percent of the world’s natural uranium.
So countries with nuclear ambitions—peaceful or not—need to first increase the proportion of U-235 in their uranium samples through a process called enrichment.
How is nuclear energy made?
In most cases, nuclear energy production begins with uranium enrichment.
Uranium enrichment most commonly occurs in gas centrifuges. After uranium is converted into gas, it is fed into centrifuges, which rotate at high speeds, to separate the slightly heavier U-238 from U-235. Each round of rotation in a centrifuge lowers the proportion of U-238 and increases that of U-235 in the sample. Uranium can be enriched to various levels, which fall into two categories:
low-enriched uranium (LEU), which has less than 20 percent U-235 and is often used for nuclear power or in non-power reactors, which produce materials for medical use, scientific research, and other purposes; and
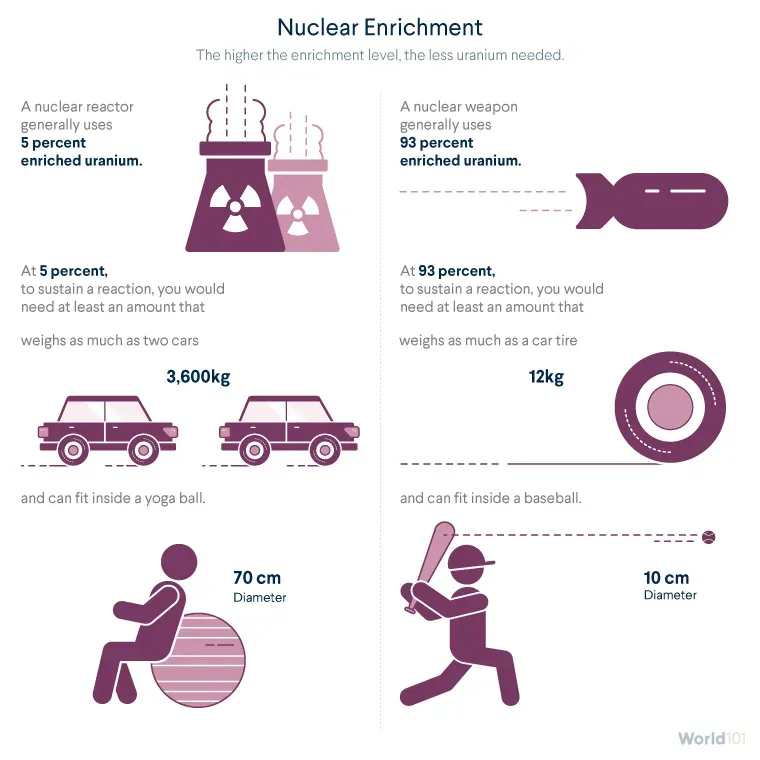
highly enriched uranium (HEU), which has 20 percent or more U-235 and is mainly used for military purposes: to develop nuclear weapons and in a few other specialized applications such as the reactors on nuclear-powered submarines.
Any level of HEU can be used for a weapon, but HEU enriched to at least 90 percent, sometimes called weapons-grade uranium, is the most common. Higher levels of enrichment mean that less uranium is required to produce a weapon. That means warheads can be smaller and lighter, enabling missiles to cover greater distances and aircraft to deliver more weapons.
How long does it take to create a nuclear bomb?
Once a country can enrich uranium, it can produce enough HEU for a nuclear weapon within months.
The materials, technology, and expertise needed for enrichment can be used to both generate nuclear power and develop nuclear weapons. Once a country is capable of enriching uranium for nuclear purposes, even peaceful ones, it usually can produce enough material for a nuclear weapon. For that reason, monitoring proliferation is exceptionally difficult.
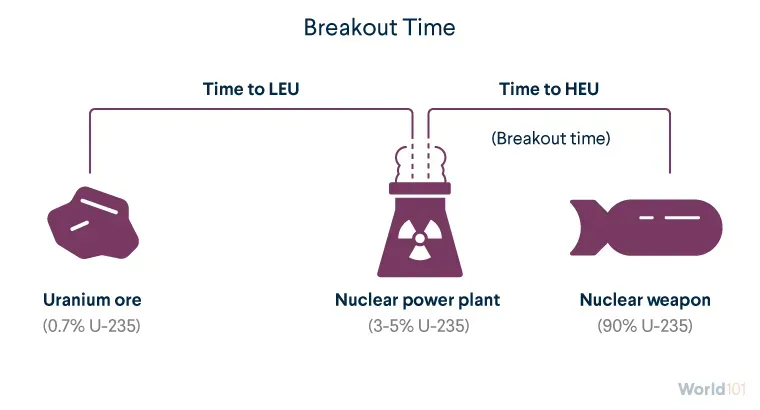
Breakout time refers to the estimated time that a country with an existing enrichment plant would need to produce enough HEU for a nuclear weapon. (Other technologies are needed to build the weapon itself.)
Enriching uranium in its natural state to between 3 and 5 percent U-235, the LEU enrichment level used in most nuclear power plants, takes a lot of time and resources. It's relatively easier and quicker to then enrich LEU to 90 percent needed for weapons-grade uranium. Once a country can enrich uranium at all, its breakout time is often just months.
And that’s a precarious prospect.
But more than one path leads to a nuclear bomb.
Producing HEU is one way to create a nuclear weapon. But uranium can also produce plutonium, an element that is not naturally occurring and is much more powerful than uranium.
Depending on the level of the isotope Pu-239, plutonium can be differentiated into
reactor-grade plutonium, containing between 55 and 70 percent Pu-239, which is extracted from spent fuel that has been irradiated, or exposed to radiation, for years in a nuclear reactor; and
weapons-grade plutonium, containing at least 90 percent Pu-239, which is extracted from spent fuel that’s been transferred to a special plutonium production reactor. That special reactor only needs weeks or months to produce weapons-grade plutonium.
Although the two kinds are created by different methods, both come from spent, uranium fuel in reactors. Weapons-grade plutonium is made specifically for military purposes, and a plutonium production reactor has a different design from a typical nuclear reactor. The presence of such a reactor is a sign that a country could be building nuclear weapons.